Abstract
Introduction: Patients with severe hemophilia A (HA) may have variable bleeding phenotypes despite having equivalently low factor VIII (FVIII) levels of <1%. The basis for this heterogeneity in the clinical expression of severe hemophilia is poorly understood. Recent evidence indicates that global assays of coagulation may be better predictors of the coagulation capacity and hemostasis compared with traditional coagulation tests. Current prophylaxis goals in patients with hemophilia minimize time spent at baseline. Thus, we hypothesized that evaluating endogenous thrombin potential (ETP) at baseline, 10% and 40% FVIII correction levels would better reflect real-world scenarios and the effects of treatment.
Our objective was to study thrombin generation (TG) parameters (ETP, peak thrombin and lag time) at baseline, 10% and 40% factor correction levels in patients with severe hemophilia A. Additionally we aimed to determine if the baseline TG parameters were predictive of response at the spiked levels.
Methods: Our study was a single institution cross-sectional study. Patients were recruited from 4/28/2011 - 7/31/2014. A total of 123 patients with HA were recruited out of which 55 patients with severe HA without inhibitors were included in our study. Descriptive characteristics of the study population included in our study are described in Table 1. A hemophilia severity score (HSS) was calculated based on adjusted bleeding scores, factor scores and joint scores. Baseline plasma samples at trough FVIII were obtained from patients and thrombin generation was evaluated using a standard calibrated automated thrombogram (CAT). Full-length recombinant FVIII was added to trough patient plasma for final factor concentrations of 0.1 U/dl and 0.4 U/dl. Spearman's rank correlation coefficients were calculated and p values of <0.05 were considered statistically significant.
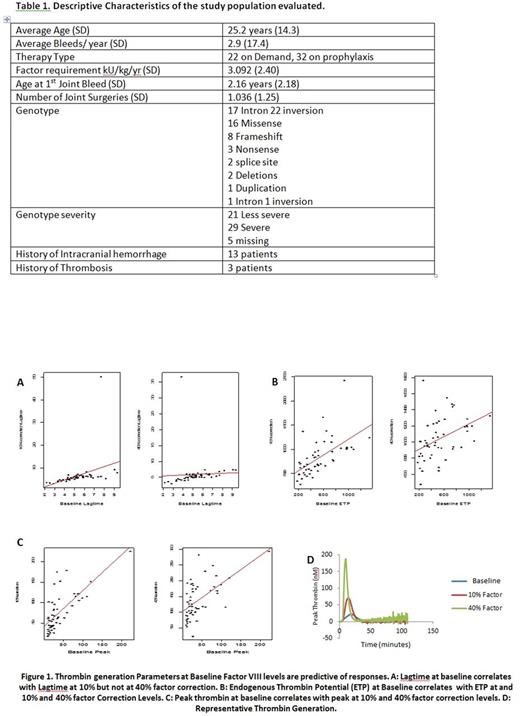
Results: Samples from 55 patients with severe HA without inhibitors were evaluated. CAT mean lag times were 5.04 minutes (SD 1.92), 8.06 minutes (SD 10.0), 5.87 minutes (SD 4.38); mean ETPs were 485.4 nM/minute (SD 296.6), 822.9 nM/minute (SD 372.2), 1038 nM/minute (SD 254); and mean peak thrombin levels were 37.2 nM (SD 37.9), 77.7 nM (SD 45.9), 138.4 nM (SD 58.5) at baseline, 10% correction and 40% factor correction respectively. There were no significant correlations between HSS score and TG parameters at baseline, 10% or 40%. The correlations ranged from -0.15 to .012. Severe versus non-severe genotype and TG was analyzed in 47 participants in whom genotype status was recorded. In the 29 patients with severe genotypes (62%) there were no comparisons that were statistically significant. However, the peak thrombin generation at 40% correction trended towards significance with levels lower in the patients with severe as compared to those with non-severe genotypes (p=0.07). Additionally we evaluated if TG parameters at baseline were predictive of responses at 10% and 40% correction levels. Baseline ETP significantly predicted ETP with both 10% (p<0.001) and 40% correction (p=0.0018) levels. Baseline peak thrombin was a significant predictor of peak thrombin levels with 10% (p<0.001) and 40% correction (p<0.001). Lagtime at baseline was a significant predictor of lag time at 10% correction (p=0.008) but not 40% correction levels (p=0.737). (Figure 1)
Conclusion: Our results confirm previous studies indicating variability in thrombin generation among patients with severe HA (FVIII<1%) at baseline as well as at different correction levels. Since phenotypic heterogeneity is observed in patients with severe HA, this may suggest that TG better correlates with bleeding severity as compared to factor levels. Even though the HSS scores do not correlate with TG parameters this may be reflective of the need for a more robust phenotypic score evaluating bleeding severity in patients with hemophilia. Additionally, baseline TG parameters appear to be responsive to treatment at 10% and 40% factor correction levels, suggesting that baseline measurements maybe sufficient to predict response. The introduction of continuous primary prophylaxis has dramatically changed the natural history of the disease as well as orthopedic status. Therefore, new criteria may be warranted to define the degree of clinical severity of hemophilia to improve patient management and healthcare costs.
Dunn: World Federation of Hemophilia USA: Membership on an entity's Board of Directors or advisory committees; Shire: Consultancy, Other: Unrestricted educational grant, Research Funding; Octapharma: Other: Unrestricted educational grant; NovoNordisk: Other: Unrestricted educational grant; Kedrion: Other: Unrestricted educational grant; Biogen: Other: Unrestricted educational grant, Research Funding; CSL Behring: Consultancy, Other: Unrestricted educational grant; Bayer: Consultancy, Other: Unrestricted educational grant; Alnylam: Other: Unrestricted educational grant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal